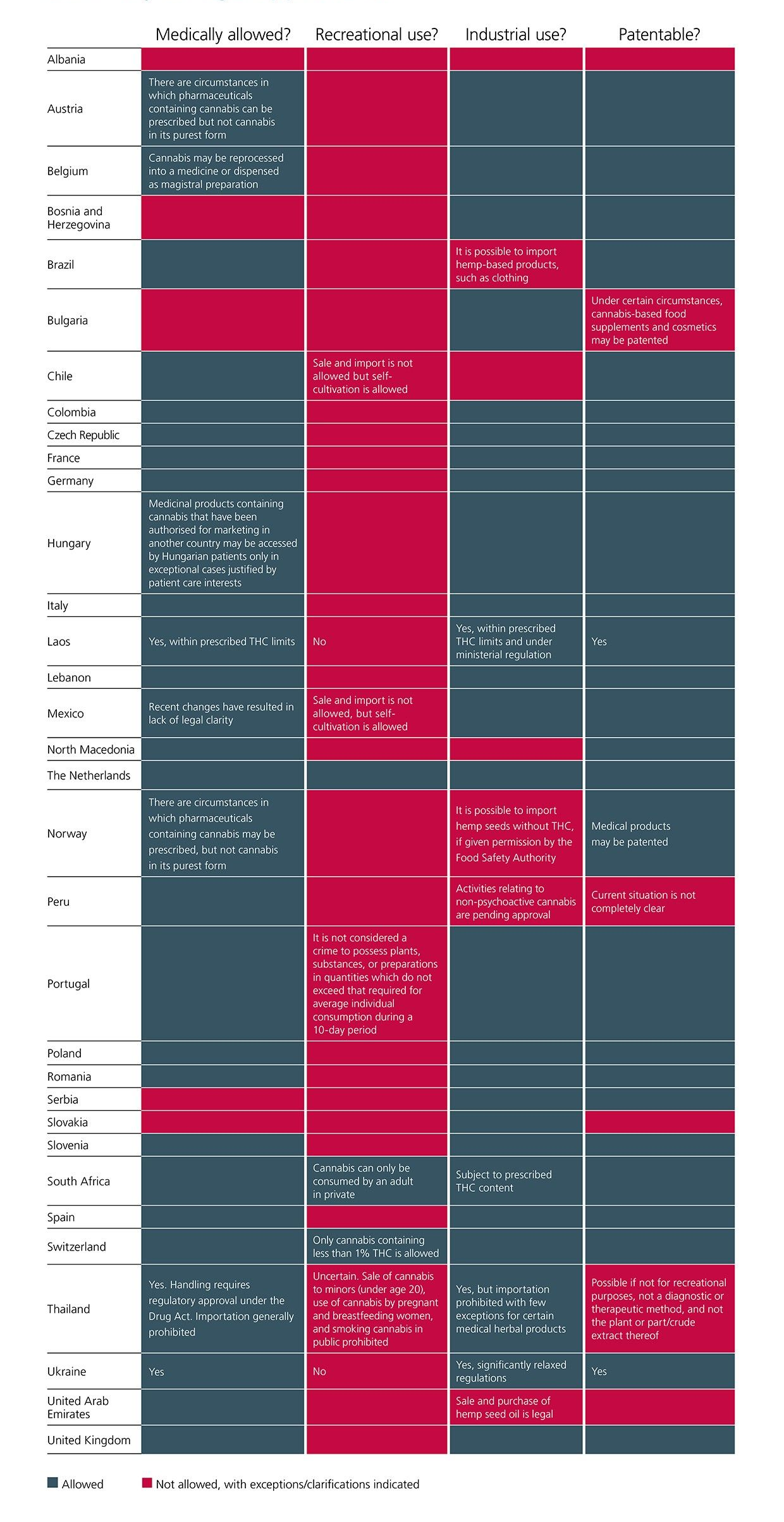
CMS Expert Guide on cannabis law and legislation gives stakeholders detailed and updated information on the latest developments in cannabis regulation in key markets. The Guide now covers 33 jurisdictions, including an additional new jurisdiction for Norway.
In Norway, cannabis is classified as a narcotic drug, which in general may not be used for medical purposes. Nevertheless, some exceptions may be applied (e.g. two prescription-only medicines have been authorised in Norway, and cannabis-related medicines and products with a THC content of less than 1% that are not approved in Norway, however, may be prescribed by Norwegian doctors).
Other developments that occurred between 2023 and the beginning of 2024 include:
- In May 2024, South Africa approved the Cannabis for Private Purposes Act that attempts, among other things, to clarify the definition of “in private” and the quantities that qualify as “private use”. Provisions on the commercialisation of cannabis are not included in this Act, but are expected in the future.
- In Poland, from August 2023, provisions strictly governing the practical prescription of medical cannabis came into force whereby a prescription for medical cannabis can be issued after the prescriber has made a verification through the electronic system, or after receiving confirmation from the patient that the amount and type of medicinal products previously prescribed to the patient in prescriptions issued and filled are not sufficient for the proper conduct of pharmacotherapy. Hence, a further prescription is necessary for the continuation of treatment and can be issued if no more than three months have elapsed since the patient was last examined.
- In February 2024, the Czech government extended the list of approved addictive and psychoactive substances like HHC. An updated list of addictive substances is expected later in 2024.
- After updating regulations in 2023, Ukraine recently adopted a new legal framework for medical cannabis, which will enhance patient access to cannabis based treatment. Ukraine also eased regulations on the cultivation, processing and use of industrial hemp. Under the law regulating the circulation of cannabis in Ukraine, which is set to take effect on 16 August 2024, the cultivation, processing, manufacturing and compounding, wholesale and retail sale, import and export are permitted for: medical cannabis; products of its processing; and cannabis substances, all solely for medical, R&D and educational purposes.
- A draft amendment is under discussion in Romania for the law governing the regulation of narcotic and psychotropic plants, substances and preparation as well as of the Pharmacy law, which introduces changes on the relevant penalty regime.
- Minor amendments have also been introduced to the chapters for Austria, Brazil, France, Hungary, Italy, Mexico, Peru, Portugal, Spain and the UK.
To view the updated CMS Expert Guide click here.
For more information on cannabis in the sector, contact us or your regular CMS experts.